Sathanandam pioneers FDA trial of minimally-invasive device for premature infants with heart defects
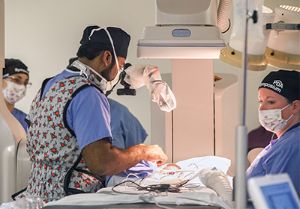
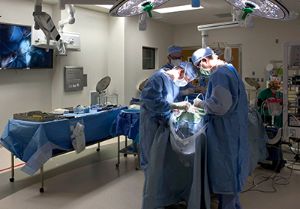
Le Bonheur Interventional Cardiologist Shyam Sathanandam, MD, FSCAI, has pioneered a new technique to close PDAsin extremely low birth weight (ELBW) infants participating in a 10-site, FDA trial for the device using the closure. That device, Abbott’s Amplatzer Piccolo Occluder, received FDA premarket approval last month to treat PDAs in ELBW infants.
The minimally invasive technique performed in the hospital’s hybrid catheterization lab was designed to shorten the length of stay for premature infants and improve the premature infant’s recovery time.
The Amplatzer Piccolo Occluder is the first transcatheter cardiac device to earn FDA approval for premature infants. The device, developed by the global health care company Abbott, is a self-expanding, wire mesh device inserted through a small incision in the leg and guided through vessels to the heart, where it is placed to seal the opening of the heart.
“We believe this will change the field of cardiology and improve care for the tiniest neonates,” Sathanandam said. “The transcatheter closure allows babies to grow and heal faster.”
To date, Sathanandam has performed 140 closures on neonates weighing two kilograms or less and played a key role in the multisite Abbott trial, which closed enrollment Feb. 1. Le Bonheur has enrolled more neonates than any other center in the study. In February, Le Bonheur Children’s Hospital implanted the first commercial FDA-approved transcatheter device for PDA patients. Shyam and his team closed the PDA in the first patient postapproval, a 2-week old baby girl, born at 28 weeks and weighing one kilogram.
Help us provide the best care for kids.
Le Bonheur Children's Hospital depends on the generosity of friends like you to help us serve 250,000 children each year, regardless of their family’s ability to pay. Every gift helps us improve the lives of children.
Donate Now