 This fall, Le Bonheur Children’s Neuroscience Institute will be the first in the world to install TRIUX™ neo, the newest generation in magnetoencephalography (MEG) technology. Le Bonheur is one of the few pediatric hospitals in the country with this level of brain mapping technology and the clinical expertise to improve outcomes for complex epilepsy patients.
This fall, Le Bonheur Children’s Neuroscience Institute will be the first in the world to install TRIUX™ neo, the newest generation in magnetoencephalography (MEG) technology. Le Bonheur is one of the few pediatric hospitals in the country with this level of brain mapping technology and the clinical expertise to improve outcomes for complex epilepsy patients.
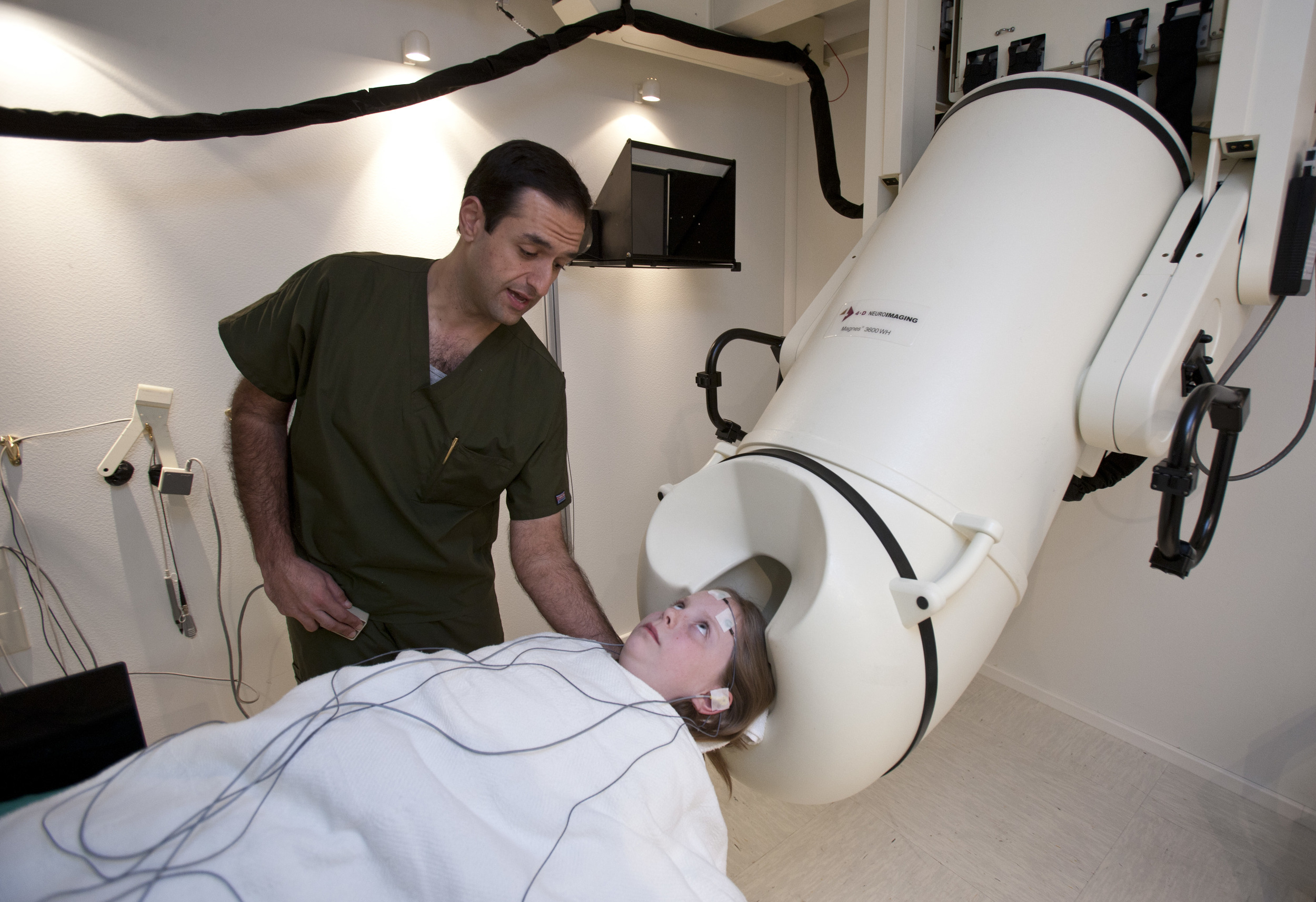
Le Bonheur began offering the non-invasive MEG testing more than 10 years ago. Since then, the hospital has assembled a comprehensive team of neuroscientists who consult with pediatric neurologists and neurosurgeons on treatment plans for complex brain tumors and epilepsy cases. The team includes PhDs with extensive expertise in non-invasive brain imaging and stimulation methods. The hospital performs about 130 MEG studies annually — around 70 percent for clinical care and 30 percent for research studies.
The team uses functional brain imaging to record sensory, motor and cognitive functions in a patient’s brain. By identifying areas of abnormal activity and those responsible for specific normal functions, the team provides information, which when combined with the structural images from MRI and CT, can help guide treatment plans. MEG is a key part of the non-invasive brain mapping capabilities at Le Bonheur. The technology has improved outcomes for children with epilepsy.
“We have developed means to test where language cortex is in children down to 1 year of age. We can localize seizure foci and can map sensory and motor areas with MEG,” said Frederick Boop, MD, chairman of the Department of Neurosurgery for the University of Tennessee Health Science Center and co-director of the Neuroscience Institute. “This has allowed us to perform surgery in children more safely and with fewer complications. It has also allowed us to extend our surgical capabilities in children with epilepsy to those who might not have been recognized as surgical candidates in the past.”
Magnetoencephalography (MEG) records neuronal responses based on the magnetic fields that are produced when groups of brain cells become active. Clinical neuroscientists interpret this information to localize the origin of magnetic fields and identify areas of the brain that are active during movement, sensation and the production and perception of speech.
The TRIUX™ neo is the newest generation MEG platform by MEGIN-York Instruments and will be operational by the end of 2018. The new technology has advancements that will be beneficial for patients who have implanted devices like VNS.
“The new system will feature state-of-the art hardware and software capabilities especially designed for the challenging populations we encounter, including previous contraindications such as implanted medical devices that precluded these patients from undergoing MEG testing,” said Roozbeh Rezaie, PhD, magnetoencephalography (MEG) lab director.
Pediatric Neurologist James Wheless, MD, says the new MEG technology will allow Le Bonheur to give parents key information for risk-versus-benefit discussions before surgery – especially those with challenging neurological disorders.
“Many children with difficult-to-control seizures have other co-morbidities and, with these, other implanted devices. These can vary from baclofen pumps to VNS therapy. In the past these children could not have a MEG study, now they will be able to, which will expand the number of children who can benefit from this diagnostic test,” said Wheless, MD, chief of pediatric neurology for UTHSC, director of the epilepsy program and co-director of the Neuroscience Institute.
In addition to MEG, Le Bonheur’s Neuroscience Institute offers all of the known diagnostic tools to identify and treat neurological disorders. The epilepsy center is a National Association of Epilepsy Center Level IV accredited center, the highest level for pediatric patients. Transcranial Magnetic Stimulation (TMS) is a non-invasive method for detailed mapping of the motor and speech areas close to brain lesions (tumors, AVMs, etc.) that could be surgically removed.
The Clinical Neurosciences team is also leading the way in developing innovative treatments. The team has published case studies highlighting non-invasive functional brain mapping as an alternative to the invasive cortical stimulation mapping. The team documented success using transcranial magnetic stimulation (TMS) in children as young as 11 months old. Their research has been published in Neurology, Journal of Clinical Neurophysiology and Open Access Journal of Neurology & Neurosurgery



