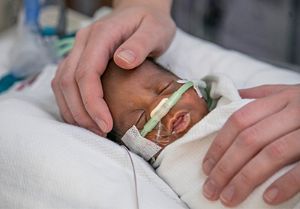
NICUSeq Study
Le Bonheur was one of five children’s hospitals to participate in a randomized clinical trial, coordinated by Illumina, Inc. scientists, to determine the effect of clinical whole-genome sequencing (cWGS) on clinical management of critically-ill newborns in the U.S. Le Bonheur Genetics Chief Chester W. Brown, MD, PhD,
led the hospital’s investigation efforts and co-authored a study with Illumina and fellow Le Bonheur and University of Tennessee Health Science Center (UTHSC) investigators. Findings show that using cWGS outperforms the current standard of care twofold for critically-ill newborns suspected of having a genetic condition, both in terms of diagnostic efficacy and change of clinical management. Findings from the “NICUSeq Randomized Time-Delayed Trial” were recently published in JAMA Pediatrics and include data supporting the widespread adoption and implementation of cWGS for critically-ill newborns.
“The NICUSeq study shows us the importance of large-scale genetic testing in newborns, leading to early diagnosis of genetic conditions and informing decision making for physicians and families,” said Brown, who also holds the St. Jude Chair of Excellence in Genetics at UTHSC.

A total patient population of 354 critically-ill newborns of diverse ethnicities were enrolled in the study, which took place at four sites in addition to Le Bonheur — Children’s Hospital of Philadelphia, Children’s Hospital & Medical Center in Omaha/University of Nebraska Medical Center, Children’s Hospital of Orange County/ Rady Children’s Institute for Genomic Medicine and St. Louis Children’s Hospital/Washington University. The newborns were randomized to receive cWGS results within either 15 or 60 days of evaluation for a suspected genetic condition, with a total observation period of 90 days. In both the early (15 days) and delayed (60 days) arms of the study, access to cWGS doubled the number of patients who received a precision diagnosis and corresponding change in clinical management.
Study findings complement a growing body of literature demonstrating that cWGS leads to more focused, and therefore improved, patient care and should be considered a primary tool when assessing critically-ill newborns with a suspected genetic condition. Results also suggest that swift, affordable access to cWGS may help reduce health care disparities by enabling greater diagnostic equity, as the study mirrored the real-world variables affecting patient care. The NICUSeq findings support the widespread adoption and implementation of cWGS as a first-line genetic test for critically-ill newborns, increasing the probability of greater diagnostic accuracy and potentially life-saving care changes.
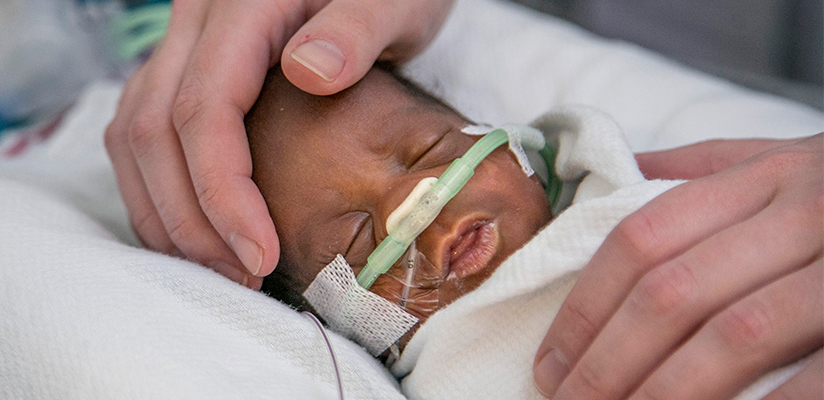
The next step is to determine how to implement the findings from the study into clinical standards of care, which poses some challenges, says Brown. He is currently part of a statewide initiative to make medical genetic services, including cWGS and other genetic testing, readily accessible to all Tennesseans with rare genetic disorders.
“Having this type of genetic information provides immediate and sustainable benefits with lifelong value, providing a genetic ‘report card’ that can be used to direct medical care throughout life,” said Brown. “We are proud that Le Bonheur and UTHSC contributed to this important effort to improve medical care for babies of the greater Memphis community.
Help us provide the best care for kids.
Le Bonheur Children's Hospital depends on the generosity of friends like you to help us serve 250,000 children each year, regardless of their family’s ability to pay. Every gift helps us improve the lives of children.
Donate Now