Heart Institute
Identifying Modifier Genes in Cardiomyopathy

Jeffrey A. Towbin, MD, Enkhsaikhan Purevjav, MD, and Lu Lu, PhD, are leading a new NIH-funded project to uncover modifier genes involved in cardiomyopathies. Unlike causal gene variants, modifier gene variants are difficult to detect using traditional genomics approaches such as genome wide association studies. Their innovative approach includes the largest mouse genetic reference population, BXD, and samples from patients with cardiomyopathies.
Cardiomyopathies are a family of heritable and acquired diseases of heart muscle that carry a high risk of heart failure and death. Mutations in several cytoskeletal genes cause cardiomyopathies. However, among patients carrying the same causal mutations, the presentation, severity and outcomes are highly variable. The high variability in cardiomyopathies indicates potential genetic modifiers, i.e., modifier genes. Modifier gene variants could interact with causal gene variants through epistasis to drive unique cardiomyopathy phenotypes.
The BXD family was founded by crossing B6 and D2 mice into 152 diverse lines. These mice are ideal for studying genetic interactions in cardiomyopathies because one parent strain (D2) exhibits cardiomyopathy phenotypes, while the other (B6) has a normal heart. BXD mice are also fully sequenced and exhibit diverse cardiomyopathy phenotypes
Using this novel approach, the investigators have chosen to focus on Z-disk myopalladin. Mutations in Z-disk myopalladin cause all major types of cardiomyopathies. Therefore, Z-disk myopalladin is a strong causal gene that can be harnessed to detect potential modifier gene variants. The investigators plan to identify modifier variants relevant to Z-disk myopalladin and create maps of genetic loci associated with cardiomyopathy phenotypes. Once candidate modifier genes are identified, the researchers will sequence samples from patients with cardiomyopathy and unaffected family members to confirm mutations or expression changes in the candidate genes.
With this project, Towbin, Purevjav and Lu aim to improve personalized care in cardiomyopathies. Identification of modifier gene variants could lead to comprehensive molecular screening and the ability to predict disease progression. Ultimately, this knowledge could help physicians make informed decisions about patient care and possibly prevent poor outcomes such as sudden death.
Non-Surgical Closure of Patent Ductus Arteriosus
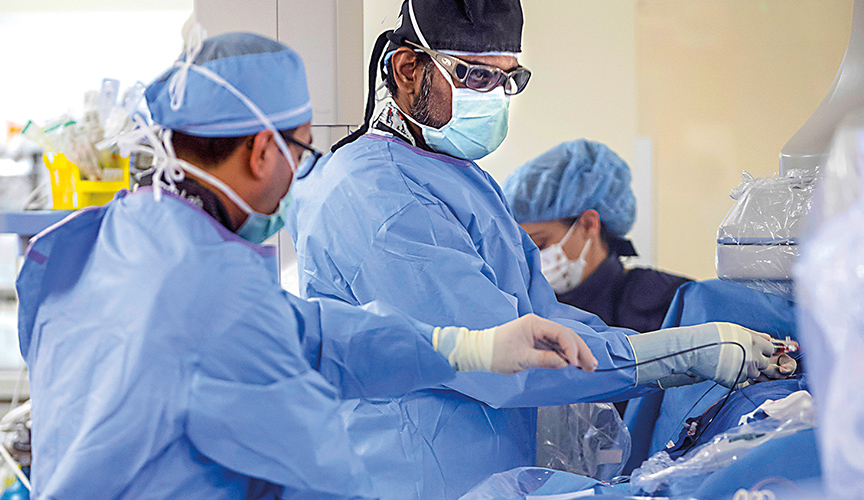
Interventional Cardiologist Shyam Sathanandam, MD, is pioneering a study on non-surgical catheterization patent ductus arteriosus (PDA) device closure in micro-premature newborns. Micro-premature newborns weighing as little as 600 grams and retaining a PDA are at high risk of developing heart failure and lung dysfunction. Therapies in the past have included medications such as indomethacin or surgical ligation, which can result in a prolonged recovery period. This study by Sathanandam and colleagues utilizes a PDA closure device to close the PDA by cardiac catheterization, limiting the potential complications of previous treatment options.
Help us provide the best care for kids.
Le Bonheur Children's Hospital depends on the generosity of friends like you to help us serve 250,000 children each year, regardless of their family’s ability to pay. Every gift helps us improve the lives of children.
Donate Now